
Winship GAMECHANGERS BLAZING NEW PATHS IN THE FIGHT AGAINST CANCER SPRING 2016 Empowering the Immune System 8 Winship’s World Reach 16 ERIC BERRY: Back in the Game 23
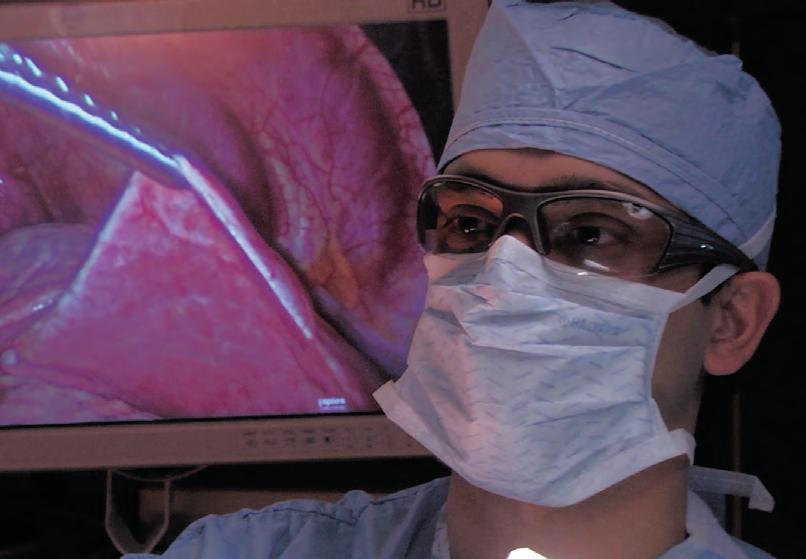
On the cover — Research discoveries that have been decades in the making are now changing outcomes for patients with lung cancer, melanoma, and other diseases. Winship has pioneered ways to find cancers earlier and treat them more effectively using targeted and immunotherapy drugs, more precise radiation therapy, and advanced surgical techniques. Here, thoracic surgeon Manu Sancheti performs minimally invasive lung surgery at Emory Saint Joseph’s Hospital using a tiny video camera.




IN THIS ISSUE
Winship in the News 2
Wally Curran debuts Winship’s strategic roadmap; new Music is Medicine exhibit; a master swimmer and survivor.

Empowering the Immune System 8
A close look at how new drugs work with a patient’s own immune system to fight cancer.
Winship’s World Reach 16
You can find Winship people at work all over the globe.
Getting the Jump on Lung Cancer 18
Better screening and minimally invasive surgery are changing the prognosis for patients with early-stage lung cancer.
Winship
Editor: Judy Fortin
Managing Editor: Catherine S. Williams
Art Director: Peta Westmaas
Photographer: Jack Kearse
Production Manager: Carol Pinto
Emory | Winship Magazine is published biannually by the communications office of the Winship Cancer Institute, a part of the Woodruff Health Sciences Center of Emory University, emoryhealthsciences.org. Articles may be reprinted in full or in part if source is acknowledged. If you have story ideas or feedback, please contact judy.fortin@emory.edu. © Spring 2016. Website: winshipcancer.emory.edu. To view past magazine issues, go to winshipcancer.emory.edu/magazine.
Number 29: Back in the Game 23
Atlanta native and a star safety with the Kansas City Chiefs, Eric Berry came to Winship to be treated for lymphoma.

Game Changers in Philanthropy 24
Grateful patients contribute to research and inspire hope for the future.
Supporting Winship Night and Day 26
Photographs from Winship’s premiere fundraising events, Fashion A Cure and the Winship Gala.
Navigating the Language of Cancer 28
New ways to help patients better understand the world of cancer treatment.
Emory University is an equal opportunity/equal access/affirmative action employer fully committed to achieving a diverse workforce and complies with all federal and Georgia state laws, regulations, and executive orders regarding nondiscrimination and affirmative action. Emory University does not discriminate on the basis of race, age, color, religion, national origin or ancestry, sex, gender, disability, veteran status, genetic information, sexual orientation, or gender identity or expression.
3
SPRING 2016
“Eric’s story and his strength are truly a testament to his individual willpower, his great spirit, his faith, and his supportive family. More than that, he is a great human being who has been very generous with his time to help our patients.”
—Christopher Flowers
23
[PHOTO COURTESY: KANSAS CITY CHIEFS]
ROADMAP TO SUCCESS
THE MOST SUCCESSFUL ORGANIZATIONS require continual renewal and focus in their journey to success. Winship is no exception, especially with its significant scale and dynamic pattern of patient care, research, and teaching. Every three to five years, Winship has undergone a rigorous process of strategic planning designed to reinvigorate its focus on developing better preventive and therapeutic measures to eliminate cancer. The current Winship strategic plan, spearheaded by Wally Curran, will serve as the roadmap to guide Winship and its priorities for the next three years.
HERE ARE WINSHIP’S GOALS:
INNOVATIVE RESEARCH
Winship will advance its status as an elite cancer center in cancer innovation and discovery as measured by its total National Cancer Institute funding.
BEST PLACE TO WORK AND LEARN
Winship will educate, train, and mentor professionals and trainees engaged in Winship cancer research and care in preparation for leadership roles in these realms.
PROVIDER OF CHOICE
Winship will strengthen its unified, researchdriven patient- and family-centered care experience in consistent delivery models which are value-driven, scalable, and sustainable.
IMPACT THE LIVES OF GEORGIANS
Winship will lessen the cancer burden in Georgia by applying its research findings to specific cancer challenges within Georgia, especially those challenges among historically underserved populations.

OPTIMAL STRUCTURE FOR SUCCESS
Winship’s financial and programmatic alignment with Emory will enable the optimal Winship structure for success and growth.
2 Winship Magazine | winshipcancer.emory.edu winship | in the news
2.
3.
4. 5.
Executive Director Wally Curran with radiation oncology nurse Jewell Hudson.
BRYAN MELTZ
1.
The Unsinkable Judith Haase

If the ship is going down, Judith Haase is the kind of person you want in your lifeboat: not only is she a great swimmer and a retired nurse with decades of experience (including stints in the Peace Corps and CARE), but she also has the indomitable spirit that would get you through a crisis. She survived her own health crisis of a stage III breast cancer diagnosis in 2013 and oh, yes, she’s also a Katrina survivor.
“Sometimes you’ve just got to push through,” says Haase. “You don’t want to, but you do it.”
Haase says she struggled with both the physical and mental aftermath of cancer diagnosis and
treatment. Enrolling in the Winship at the Y program helped get her back in the exercise groove and talking with Winship psychiatric oncologist Wendy Baer “saved my life.” She kept swimming throughout treatment. She says it helped alleviate side effects like lymphedema and continues to induce a meditative state that acts as a mental salve.
Now 75, Haase volunteers two days a week at Emory Saint Joseph’s Hospital, which is where she got her chemo and radiation treatments, and she swims three days a week. Ten years ago, she was recruited to swim in U.S. Masters competitions both as an individual and as a member of Killer Whales, a Georgia team whose motto is “The older we get, the faster we were.” She’s determined to qualify for the Senior Games in 2017 to be held in Birmingham, Alabama. W


Winship Magazine | spring 2016 3
WINSHIP CANCER SERVICE LINE
As part of its new cancer service line, Winship Cancer Institute and Emory Healthcare have made two important appointments: Melissa Childress (pictured right) is vice president of cancer services and Kim Slusser (pictured left) is vice president of cancer nursing. The Winship cancer service line will facilitate the further development of a culture of excellence in quality and service across all inter-professional cancer care teams.

Childress is responsible for coordinating cancer services along with hospital and clinic leadership across multiple hospitals within Emory Healthcare. The goal is to have a Winship clinical care model that is consistent and seamless, patient and family focused, and research driven for all cancer types across all locations.
Slusser is responsible for the development and oversight of Winship oncology nursing practice guidelines across Emory Healthcare to enhance the continuum of care and lead the ambulatory clinical care teams for the Emory system. She leads clinical quality, safety, outcomes, education, and oncology nursing research. W
5 YEARS RUNNING!
Winship cancer network
October 3, 2015
Winship Cancer Institute and the Lewis Hall Singletary Oncology Center at Archbold Memorial Hospital in Thomasville, Georgia, have teamed up as part of the Winship Cancer Network. Archbold became the first hospital outside Emory Healthcare to join this elite group of health systems and community hospitals known as Emory’s Winship Cancer Network. The partnership will enhance access to state-of-the-art cancer research and treatment for patients.
The Winship Cancer Network was created to improve access to high-quality cancer care, the newest research, and continuing patient and provider education for affiliate partners in the state and around the Southeast region.

Patients will also benefit from expedited second opinions and access to clinical trials. W
winship5k.emory.edu

winship | in the news
NEW WINSHIP LEADERSHIP APPOINTMENTS

Suresh Ramalingam, an internationally recognized lung cancer physician-investigator, has been named deputy director of Winship Cancer Institute. He also serves as assistant dean for cancer research in the Emory School of Medicine.

In his role as Winship’s deputy director, Ramalingam leads the integration of the research, clinical, and educational components within Winship. This position was previously held by Fadlo Khuri, who assumed the presidency of American University of Beirut.
Ramalingam, a professor in Emory’s Department of Hematology and Medical Oncology, serves as Winship’s director of medical oncology and the Lung Cancer Program. He co-leads Winship’s Discovery and Developmental Therapeutics Program.
Ramalingam is recognized for his research in developing individualized therapies for patients with small cell and non-small cell lung cancer.
Sagar Lonial is the new chair of the Department of Hematology and Medical Oncology within Emory University School of Medicine and Winship Cancer Institute.

Lonial, an expert in the biology and treatment of patients with multiple myeloma, also serves as Winship’s chief medical officer and as professor of hematology and medical oncology.

Lonial’s research focuses on combinations of novel agents in myeloma therapy and development of new targets and treatment strategies for high-risk myeloma. He directed two large studies of monoclonal antibodies which led to Food and Drug Administration (FDA) approval. His team has contributed to all the major drug approvals in myeloma over the past decade, and he is currently leading a global genome sequencing study in newly diagnosed myeloma.
Mylin Torres, an associate professor in the Department of Radiation Oncology, is the new director of the Glenn Family Breast Center at Winship. The center is dedicated to improving the treatment of patients with breast cancer through the careful align ment of research with patient-centered care.

Torres, a board certified clinician and researcher, focuses her investigations on clinical trial development, outcomes measures, identifying patients at risk for side effects of breast cancer treatment, and quality of life metrics for breast cancer survivors.
David Edwards is the new senior director of development for Winship Cancer Institute. He joins Winship from the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, where he served as director of major gifts. In addition to leading Winship’s fundraising efforts, Edwards works closely with Winship faculty and leadership, the Winship Advisory Board, Friends of Winship, and the Woodruff Health Sciences Center development team.
Winship Magazine | spring 2016 5
MICHAEL HEAPE
MUSIC IS MEDICINE EXHIBIT
Platinum records, autographed guitars, and hand-written lyrics from some of the biggest names in the music industry are part of a priceless collection of memorabilia donated to Winship Cancer Institute by Joel A. Katz, a global entertainment and media attorney of Greenberg Traurig, LLP in Atlanta. The Joel A. Katz Music is Medicine Collection, now on display at Winship and open
to the public, is considered one of the most musically diverse collections ever assembled in any hospital. The gift coincides with the T.J. Martell Foundation’s establishment of The Joel A. Katz Music is Medicine Fund, which provides support for innovative cancer research at Winship.
Patients, caregivers, and visitors have the rare opportunity to view the three dozen items on display including multi-
platinum commemorations of worldwide record sales for Michael Jackson, Julio Iglesias, and Georgia native James Brown; guitars signed by Paul McCartney, Willie Nelson, and George Strait; a 2012 Grammy nominees award plaque; and handwritten lyrics from country star Alan Jackson. The comprehensive collection of music history spans decades and genres including rock, pop, hip-hop, and country. W




6 Winship Magazine | winshipcancer.emory.edu winship | in the news
Joel A. Katz (above left) talks with Wally Curran on opening night of the exhibit.
The collection is located on the Tunnel level of the Winship building on the Clifton campus.
The spirit of Winship
Atlanta remembers Robert L. “Bobby” Rearden as a successful businessman and a member of the bid team that brought the 1996 Centennial Olympic Games to Atlanta. Winship remembers him as a steadfast supporter and a man of great spirit and compassion.
Rearden passed away in January 2015 while undergoing treatment for a recurrence of leukemia, and in his honor the Robert L. “Bobby” Rearden Spirit of Winship Award was launched to recognize those who demonstrate exemplary service, teamwork, and a commitment to public good. The first recipients of the

Sanda to lead prostate cancer biomarker study

The Emory, Harvard, and University of Washington Prostate Cancer Biomarker Center has received a $4.2 million grant from the National Cancer Institute’s Early Detection Research Network to develop multiplex tests for detecting prostate cancer and improving survival benefits. Winship member Martin Sanda, chair of the Department of Urology, is the principal investigator of the multi-center collaboration. He is joined at Emory by Eugene Huang, professor of biostatistics and bioinformatics at Rollins School of Public Health; David Schuster, director of the Emory Division of Nuclear Medicine and Molecular Imaging; and Carlos Moreno, associate professor in the Emory Departments of Pathology and Laboratory Medicine and Biomedical Informatics. W
award are Jessica Neely, a physician assistant in the Department of Hematology and Medical Oncology, and Julie Whitehead, a volunteer in the Winship Patient and Family Resource Center. Neely and Whitehead were chosen because they exemplify Winship’s culture of caring.

The Spirit of Winship Award will be given annually to a faculty or staff member and a volunteer and will include a monetary award to be made in the name of each award recipient to an area of their choosing within Winship. W
Novel therapy programs cancer cell death
Genetic mutations in lung cancers make cancerous cells in the body resistant to standard treatment therapies such as chemotherapy and radiation. Winship cancer biologist Xingming Deng (pictured above right) and his lab have identified two novel agents that could reverse the resistance of cancer cells to treatment.
Deng is the principal investigator of two R01 grants from the National Institutes of Health aimed at investigating these agents, and totaling over $3.6 million. Deng is hoping that a new class of anti-cancer agents will lead to the improvement of lung cancer outcomes and survival. If successful, Deng will have developed two novel targeted therapies for lung cancer as well as for other common malignancies such as multiple myeloma, leukemia, and breast cancer. Deng anticipates these new lines of therapy will be ready for clinical trials for patients at Emory by 2020. W

Winship Magazine | spring 2016 7
Robert Rearden III, Jessica Neely, Julie Whitehead, Dell Rearden and Alison Rearden Murrah
Bobby Rearden
New immunotherapy drugs can help signal the immune system to attack. Here an individual T cell (blue) destroys a cancer cell.

8 Winship Magazine | winshipcancer.emory.edu feature | immunotherapy
EMPOWERING THE IMMUNE SYSTEM
By Sylvia Wrobel n Medical Illustrations by Michael Konomos
A decade ago, there were few effective treatment options for patients with advanced melanoma or lung cancer. That’s changing, thanks to new immunotherapy drugs which can unleash a patient’s own immune system. An Emory scientist’s groundbreaking discoveries pointed the way to the new therapies, and Winship clinical investigators have been involved in clinical trials of almost every immunotherapy drug approved by the FDA. While patients benefit from an increasingly wider range of treatment options, Winship clinical and laboratory investigators take on the challenge of making these therapies even more effective.

LUNG CANCER
In 2014, Danny Foshee experienced a sudden onset of extreme chest pain. He thought it was his heart, but a CT scan identified abnormal spots on his right lung. Biopsy results showed non-small cell lung cancer. His local oncologist ordered a PET scan and tumors lit up like Christmas tree lights. The largest was the size of a baseball. With cancer too advanced for surgery, a clinical trial was recommended. Soon after, Foshee met with Suresh Ramalingam, Winship deputy director and director of medical oncology, and
Rathi Pillai, the medical oncologist who would become Foshee’s primary Winship physician. After reviewing his case in detail, they asked Foshee if he would like to be considered for a clinical trial under way at Winship that compared the effectiveness of traditional chemotherapy— which directly attacks cancer cells but also other fast-growing cells in the body, such as hair follicles—and a new immunotherapy treatment designed to strengthen the immune system’s ability to recognize and fight cancer cells.
Winship Magazine | spring 2016 9
Rathi Pillai with her patient Danny Foshee.
feature | immunotherapy
He was enthusiastic, as was his referring physician, but to be eligible for the appropriate trial, he needed to have one of three biomarkers on his tumor cells. Results from the first biopsies were negative. Then, only days before the trial closed to new patients, he tested positive for high levels of PD-L1.
As Ramalingam and Pillai explained to Foshee, producing the protein PD-L1 is one way cancer outsmarts the immune system. On non-cancerous cells, PD-L1 signals the immune system not to attack. That can be very useful, for example, during pregnancy when PD-L1 plays an important role in immune system tolerance of the fetus. But when cancer cells produce PD-L1, its presence on the cells tricks the immune system into not recognizing an enemy that should be attacked. Patients with high levels of PDL1 in tumor cells—and in the immune cells surrounding lung tumors—often do not respond as well to treatment.
The good news for Foshee, however, was that his high levels of PD-L1 made him eligible for a clinical trial studying nivolumab, a new drug which blocks PD-1, the receptor to which PD-L1 binds (see Ahmed sidebar page 14). The study asked: in patients with high PD-L1 levels, would
immunotherapy with a PD-L1 inhibitor work better than chemotherapy?
Participants were randomly assigned to either immunotherapy or chemotherapy. Foshee drew chemotherapy, which meant he would receive the same drugs given patients outside of clinical trials. At first, he responded beautifully. Tumors shrank quickly. For a particularly large

Food and Drug Administration (FDA) approved nivolumab for patients with metastatic non-small cell lung cancer resistant to chemotherapy, which described Foshee to a T. (Now known by its trade name Opdivo, nivolumab had been approved for patients with advanced melanoma a few months earlier.) Ramalingam, a national leader in clinical trials for lung cancers, led one of the two trials that spurred this go-ahead for lung cancer. Several other drugs recently approved for lung cancer also have gone through clinical trials at Winship under his direction.
When the Foshees see ads for Opdivo on television, they justifiably feel pride in helping move such studies forward. They have a lot of company. Winship doctors see roughly 700 new patients a year with newly diagnosed lung cancer, and an increasing number of these patients are treated with immunotherapy, many in clinical trials. A re-energized Foshee is an inspiring example of why clinical trials are so important in the fight against cancer.
one pressing painfully on his spine, radiation oncologist Kristin Higgins recommended and delivered a course of radiation treatments. That tumor shrank in response to the therapy.
Then, after two treatments, chemotherapy stopped working. The tumors took off again.
Being in the clinical trial gave Foshee another option. He was switched to the study’s immunotherapy arm. Every two weeks for the past year, he and his wife Coopie have driven almost three hours from Vidalia in South Georgia to Winship’s infusion center. Two months after immunotherapy began, his tumors had reduced in size by half. Ongoing scans show increasing shrinkage and no spread of cancer. His worst side effect has been some itching.
While the trial was still going on, the U.S.
Use of immunotherapy is likely to expand for lung cancer patients, says Ramalingam. Certain lung cancers are among the most responsive, equaled only by melanoma. Lung cancer has more mutations than many other cancers, providing more protein “hooks” for the immune system to recognize and respond to.
While new drugs like nivolumab are game changers for patients like Foshee, there are many more patients for whom the drugs are not effective. Ramalingam’s goal is to develop biomarkers to predict which patients will respond to which drugs and what can be done to turn nonresponders into success stories. He also wants to change the fact that immunotherapy currently is used only in advanced cancers. He leads the Thoracic Cancer Committee of the ECOG-ACRIN Cancer Research Group, a national federally
10 Winship Magazine | winshipcancer.emory.edu
Patients receive immunotherapy drugs like Opdivo in intraveneous solutions delivered via infusion.
funded organization that is launching a large clinical trial of immunotherapy to prevent recurrence in patients whose early lung cancer can be removed by surgery.

That is just the kind of thinking Wally Curran, executive director of Winship Cancer Institute, wants to see happen. Curran says science has understood for decades that the patient’s own immune system has the potential to control cancer. The earliest agents, like interleukin, were meant to stimulate and add to the immune system. This approach was effective to varying degrees but often with very high toxicity. In the last decade, he says, “we have discovered how to unlock the patient’s own immune system, enabling T cells to identify cancer as a foreign agent—and we are doing it with less or no toxicity.” He’s excited by how fast the field is moving, but as amazing as the recent breakthroughs have been, and as proud as he is of Winship’s role in
bringing new drugs to market, he and his Winship colleagues aren’t yet satisfied. Every advance brings new questions, such as why drugs work only for certain subtypes of cancers, and how responses can be made more durable. Today, says Curran, Winship is doing everything it can—by supporting new programs, acquiring new technology, and recruiting and mentoring new clinician-scientists—to make sure new breakthroughs come quickly.
MELANOMA

Twice Cathy McCoy was hours from surgery when things changed. Two years ago, at a local hospital in the Tennessee mountains, she had been prepped for gallbladder surgery when a last-minute endoscopic exam revealed that the blockage of her bile duct—the source of her pain and jaundice—was not gallstones. At Vanderbilt, tests showed melanoma, an old enemy McCoy
had successfully faced three times before with skin surgery. This time, the melanoma was internal, not on the skin. Surgery was scheduled the following week. But that morning, instead of being wheeled to the operating room, McCoy again
IMMUNOTHERAPY
Current immunotherapy treatments work in three distinct ways:
• Ramping up the immune response. The first immunotherapies, cytokines such as interferon, stimulate the growth of immune cells.
• Boosting targeted therapy. The overall immune response is strengthened when immunotherapy is combined with some targeted therapy drugs.
• Releasing the brake on the immune response. Cancer cells can produce a protein that turns off the immune response. Blocking this protein releases the brake and enables the immune system to attack the cancer cells.
Winship Magazine | spring 2016 11
Cathy McCoy with husband Joe, her doctor Ragini Kudchadkar, and research coordinator Cabell Eysmans. McCoy was the first melanoma patient to complete Winship’s combination therapy study.
PETE WINKEL
found a surgeon at her bedside. We aren’t going to operate, he said. The previous day’s scans had shown not one mass but six, spread across her lung and lymph nodes. Referred to a Vanderbilt oncologist, McCoy cut to the chase. “What can be done?” Pause. “We have found,” he answered, “that traditional chemotherapy isn’t effective for metastatic melanoma. But there have been successes with immunotherapy. If you are willing to try it, I will find you the most appropriate clinical trial in the best hospital possible.” “And if I choose not to try?” she asked. This time he didn’t hesitate. “With that many masses, maybe nine months.”
As a medical resident in the early 2000s, Ragini Kudchadkar decided to focus on advanced melanoma. Friends tried to discourage her. There was little that could be done, they argued, once melanoma cells escaped the original site. And indeed, in her first years in practice, Kudchadkar spent a lot of time talking to patients about end-of-life issues. But she was determined to take part in developing drugs for such patients, and during the past decade, with the advent of new targeted and immunological therapies, her conversations with patients like McCoy are often very different.
When the McCoys arrived in Kudchadkar’s Winship office in December 2014, they had just learned their only son Tyler and his wife Alexis were expecting a baby. The clock was ticking. Please, she said to Kudchadkar, please keep me alive long enough to see my grandbaby.
Fortunately, McCoy was a perfect fit for a study at Winship, one of almost 20 for advanced melanoma. Earlier clinical trials at Winship and other leading National Cancer Institute cen-
for all patients. Go home and think about it. They shook their heads. Joe said, “We’ve already been through a lot. We are out of options. We know what we need to do.” Cathy signed. A week later, they were back for her first infusion.
Kudchadkar knew the drugs well. She and Lawson had been involved in the trials that led to ipilimumab’s approval for melanoma in 2011 and nivolumab’s in 2014. In fact, they have been involved in trials for every targeted and immunotherapy drug currently approved for melanoma.
In fall 2015, the therapy given to McCoy became the first-ever combination immunotherapy approved by the FDA for use outside of studies.

ters had found that the immunotherapy drugs nivolumab and ipilimumab, given by themselves, produced strong response rates in some—but not most—patients with metastatic melanoma. Then a Memorial Sloan Kettering study tested the two drugs given together, achieving a response in more than half of patients. The finding was confirmed by studies at other leading centers, including one headed by David Lawson, director of Winship’s melanoma team. The results were so good that even though the official trial ended in early 2014, a few top centers were given continued access to the drugs. Kudchadkar was heading the expanded access study at Winship.
The McCoys listened carefully to an explanation of the study’s possible risks and benefits. It’s not a panacea, said Kudchadkar. It doesn’t work
McCoy made the 500-mile round trip to Atlanta every other Friday for months, often accompanied by a family member or friend. It wasn’t easy. Side effects included rash, fatigue, and severe headaches. Kudchadkar brought in an endocrinologist, and McCoy soldiered on. In January 2016, proud of her contribution to the combined therapy study, she “graduated,” ringing the bell that celebrates a patient’s last infusion.
Immunotherapy drugs are so new that it’s hard to talk about long-term results, although Kudchadkar has patients from the original ipilimumab trials who have been doing well for approximately 10 years. As for McCoy, she counts every day an “abundance and blessing,” especially since being able to babysit granddaughter Presley, born in September 2015.
When the treatment of well-known patients makes the news, Kudchadkar’s patients sometimes ask if they should be considered for the therapies they just read about. She smiles.
“You got that five years ago when it was still in a clinical trial,” she answers. W
12 Winship Magazine | winshipcancer.emory.edu
feature | immunotherapy
Individualized prescriptions of immunotherapy drugs are mixed in a sterile environment in a specialized pharmacy.
FLIPPING THE SWITCH ON IMMUNE CELLS
LUNG CANCER TRICKS T CELLS
When cancer invades lung tissue,T cells come to the rescue. But some cancer cells know how to produce a protein (PD-L1) that bonds with the T cells’ PD-1 receptors and turns off the immune response. Exhausted T cells stand down and let the cancer grow.
EXHAUSTED
CHECKPOINT INHIBITION
PD-1 and PD-L1 inhibitors block this interaction and cause the body’s immune cells to recognize the cancer as an invader.
IMMUNE CELLS DESTROY CANCER
Taking the brake off the T cells reactivates their immune response, allowing them to attack and destroy the cancer.

T CELL ACTIVATED T CELL ACTIVATED T CELL CHECKPOINT INHIBITOR LUNG CANCER LUNG CANCER
LUNG CANCER CELL DEATH
PD-1
PD-1
PD-L1
PD-L1
How the PD-1 Story Is Changing Cancer Treatment
Development of the newly approved immunotherapy drugs taken by Foshee, McCoy, and other patients was greatly facilitated by the work of Rafi Ahmed, director of the Emory Vaccine Center and a Winship researcher. Ahmed didn’t set out to study cancer. Internationally recognized for work on immune memory, he had sought to understand why memory T cells, so quick to respond to and remember acute infections, did not do well battling chronic infections like hepatitis C or HIV (human immunodeficiency virus). Accepted wisdom was that ongoing infection wiped out T cells. Not so.
In 1998, Ahmed and then post-doctoral fellow Allan Zajac showed that in chronic infections the virus-specific T cells were still there but were “functionally exhausted.”

In 2006, he and then graduate student Dan Barber discovered why: the central mechanism underlying functional exhaustion is high expression on the T cells of the inhibitory receptor PD-1,
Checkpoint inhibitor drugs (represented in green above) block the PD-1, PD-LI interaction.
often referred to as an immune checkpoint. The signal that tells a T cell to turn off the immune response occurs because of an interaction between the PD-1 receptor and a protein called PDL1 produced by many normal cells. Chronic infection, the Ahmed team discovered, not only keeps stimulating the immune system (the presence of pathogens turning ON the immune response) but also increases levels of PD-1 on the T cells and PD-L1 on the infected cells (turning OFF the immune response). This continuous cycling
exhausts the T cells fighting the virus infection. That was the brake on the immune system. Next, the Ahmed team showed that blocking PD-1 receptors removed that brake, an action which rejuvenated exhausted T cells and put them back to work.

After the Ahmed publication, scientists across the country found exhausted T cells in humans with chronic infections. Cancer investigators made their own observations: T cells infiltrated tumors but didn’t attack. Cancer cells had figured out how to use the PD-1 checkpoint to their own advantage by producing high levels of PD-L1, slamming the brake on T cells’ ability to kill them. The discovery brought new energy to cancer immunotherapy
research, especially development of PD-1 blocking drugs that would release that brake.
Ahmed now collaborates with Winship clinical investigators. Suresh Ramalingam, Rathi Pillai, and he look for biomarkers in blood to tell how well PD-1 inhibitors are working in patients with metastatic lung cancer. Ahmed and radiation oncologist Mohammed Khan are exploring strategies that follow radiation therapy with a drug that blocks PD-1. In a current Winship clinical trial for melanoma and lung cancer patients with brain metastases, the team hopes the combination means the response of the tumors in the brain to radiation will be enhanced by stronger immune responses. W
14 Winship Magazine | winshipcancer.emory.edu feature | immunotherapy
Rafi Ahmed is director of Emory’s Vaccine Center and a Winship researcher.
A year in the life of Jimmy Carter
“I’ve benefited from better detection technology and treatments that didn’t exist only a few years ago...
I didn’t have to leave Georgia to get these advanced treatments because Winship, as Georgia’s only National Cancer Institute designated cancer center, has been on the front lines of breakthroughs.” —President Carter
The treatments that former President Jimmy Carter refers to included minimally invasive surgery, stereotactic radiation, and a brand new immunotherapy drug. It’s been barely a year since Winship doctors removed a tumor on the President’s liver and found four additional tumors on his brain.
As cancer survivors know, a lot can happen in a year.
Molecular testing done on the liver tumor led to a diagnosis of metastatic melanoma. President Carter shared that information with the world via a packed press conference at The Carter Center on August 20, 2015. He also told the assembled media that his doctors had recommended an advanced radiation treatment that would target the brain tumors, and an immunotherapy drug recently approved for treating metastatic melanoma.
After revealing his cancer diagnosis and treatment plan, President Carter shared his progress by giving regular updates
to his Sunday school class in his hometown of Plains, Georgia. In December, he told his church class that a recent brain scan showed no sign of cancer. In early March, he told them he no longer needed immunotherapy treatments.
The open and candid way in which President Carter has shared his experience has brought public attention and awareness to the progress being made in cancer treatment. Winship scientists and doctors are proud to be doing the research leading to better treatments and grateful that President Carter has acknowledged their role in this decades-long effort.
In recognition of scientific progress and the role Winship has played in advancing cancer research and treatment, President Carter videotaped a message that was played at the Winship Gala held on April 30th. To see the full video, go to winshipcancer. emory.edu/magazine W


Winship Magazine | spring 2016 15
President Carter recorded a video message about the progress of cancer research.
Winship’s World Reach



Winship plays a vital part in the worldwide effort to better detect, prevent, and treat cancer. Here is just a sampling* of the places and organizations where Winship clinicians and researchers work alongside international colleagues to defeat cancer.


1 4 5 6 7 8 9
Illustration by Don Morris
1. Atlanta: American Cancer Society, CDC, Georgia Center for Oncology Research and Education, Georgia Tech.
2. Washington, DC: American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, US Dept. of Defense, NASA, National Cancer Institute.
3. Philadelphia: American Association for Cancer Research, ECOG-ACRIN Cancer Research Group, NRG Oncology
4. Chicago: American College of Surgeons, College of American Pathologists, International Chemical Biology Society, Society of Surgical Oncology.
5. LosAngeles: Children’s Oncology Group, International Myeloma Society.
6. São Paulo, Brazil: National HPV Immunization Campaign.
7. Shanghai, China: Smoke-free home policy research.
8. Beijing, China: Peking University.
9. Chennai, India: The Center for Cardiometabolic Risk Reduction in South Asia Surveillance Study.

10. Haifa, Israel: Middle East Cancer Consortium.
11. Johannesburg, SouthAfrica: Chris Hani Baragwanath Hospital.
12. Tbilisi, Georgia: Tbilisi State Medical University.
13. Geneva, Switzerland: Union for International Cancer Control.
14. Aarhus, Denmark: Aarhus University.
15. Toulouse, France: International Associated Laboratory.
*For a more complete listing, go to winshipcancer.emory.edu/WinshipWorld. 2 3 10 11 12 13 15 13 14

18 Winship Magazine | winshipcancer.emory.edu
EARLY DETECTION, EARLY REMOVAL

Getting the jump on lung cancer
By Quinn Eastman
We breathe in and out, every minute of every day. Our lungs are critical for life. Yet if a group of cells in someone’s lungs starts growing into a tumor, that person usually can’t see it or feel it—until it becomes large enough to be dangerous.
The lungs are encased in the ribs, with few nerve endings. So a tumor has to grow quite large before it starts to take away enough lung capacity to cause discomfort or make someone cough. Even below that threshold, as a tumor becomes larger, it is more likely for some cells to separate off and metastasize.
Early detection of lung cancer by imaging offers an opportunity to catch a tumor before it grows and spreads. In 2011, the National Lung Screening Trial, involving more than 50,000 participants, established the life-saving value of lung cancer screening by lowdose CT (computed tomography) for people with a history of heavy smoking. In the last two years, both Medicare and private insurance began to cover the screening procedure.
Winship Magazine | spring 2016 19

20 Winship Magazine | winshipcancer.emory.edu feature | new strategies
PREVIOUS PAGE: Winship thoracic surgeons Felix Fernandez, Seth Force, Allan Pickens, Manu Sancheti.
THIS PAGE: Minimally invasive surgery allows Sancheti to remove lung tissue without opening the patient’s chest.
“Better screening is changing the outcomes for lung cancer patients by allowing us to accurately find these tumors earlier,” says Allan Pickens, a Winship thoracic surgeon and director of minimally invasive thoracic surgery and thoracic oncology at Emory University Hospital Midtown. “When we find these tumors earlier, they are generally of a smaller size and have not had the chance to spread to other parts of the body, lymph nodes, or other organs.”
Because of more screening, doctors are increasingly discovering lung cancers when they are small: in many cases, less than two centimeters wide. Clinical studies indicate this is a point when it may be possible to treat the cancer by surgery alone. In addition, surgeons have been shifting to minimally invasive approaches, also known as video-assisted thoracic surgery.
Becky Huff had been seeing radiologists, not primarily for her lungs, but to follow up on findings of calcification after a mammogram. As a result, in a CT scan of her breasts, nodules were detected in her lungs. Now 67, she quit smoking more than two decades ago, and wonders whether working in a smoke-filled office also contributed to her cancer risk.
For two years, doctors at Emory, led by pulmonologist Gerald Staton, monitored her lungs with additional CT scans every six months. Then, a change in the appearance of the nodules, along with an inconclusive biopsy, led her to consult Pickens. Before surgery, a different type of imaging—a PET scan—was performed to gauge the possibility that cancer had spread.
“To me, that was another safeguard that they knew precisely what they needed to do beforehand,” Huff says.
Using two small incisions on the side of her body, Pickens removed the upper lobe of her left lung. Two months later, in a similar procedure, he removed a segment from her right lung. When pathologists examined the removed tissue and samples from her lymph nodes, they detected no signs that the tumors had infiltrated the lymph nodes. That meant she could forgo chemotherapy and radiation.
“This is an example of when we were able to get there early, before the cancer has progressed,” Pickens says.
To be sure, her recovery from the surgeries included some pain. She had trouble finding a comfortable sleeping position, and she needed to take pain medicine for a couple of weeks. However, she had avoided surgeries that would open the chest.
“I did get over the surgery a lot quicker than other people that I’ve seen,” Huff says.
Around the time of her surgeries in the spring of 2011, Huff had begun taking piano lessons. While raising five children, she
had always wanted to learn to play. Now, five years after her surgeries and a reassuring PET scan this year, she continues to learn piano and stays active with frequent walks on her family’s wooded property in Talbot County, Georgia.
Careful imaging
Although Huff’s tumors were discovered before the National Lung Screening Trial’s results were announced, her experience illustrates how careful imaging and minimally invasive surgery are changing treatment decisions for patients with early-stage lung cancer.
Careful imaging is needed because suspicious findings on lung CT, by themselves, do not mean a biopsy or surgery is required. “Notice that we call them nodules, not tumors, at the beginning,” says William Auffermann, a cardiothoracic radiologist at Winship. “Rather than pull the alarm right away, we take a tiered approach, depending on the size of the nodule and the patient’s history.”
Some nodules are visible on CT, despite being so small that they are difficult to accurately biopsy. Radiologists describe other lung nodules as having the appearance of ground glass, and they can be tricky to diagnose. Past infections or inflammation can alter lung tissues enough so that they look suspicious, Auffermann says.
He and his colleagues have been using the Lung-RADS system, developed by the American College of Radiology in 2015. Under these guidelines, only if a nodule becomes larger than about eight millimeters on repeated imaging is a PET scan or biopsy called for. Below that size, the guidelines call for follow-up scans in three or six months.
Gold markers in puffy tissues
The majority of lung cancer surgeries are now performed using minimally invasive approaches—above 80 percent at Emory. This presents advantages to the patient: less muscle is cut and recovery is quicker. Traditionally, however, surgeons would need to touch the nodule to find it, and accessing the lung via smaller incisions prevents that.
Remember that the lung tissue is normally filled with air—sort of like a puffy sleeping bag. When someone gets a CT scan and a nodule is detected, the air is present. During surgery, the tissue collapses, causing the nodule to shift away from where it was.
At Winship, cardiothoracic surgeons Manu Sancheti, Seth Force, and colleagues have been developing a technique of using gold markers, called fiducials, to keep track of small nodules. They published their findings in 2014 in the Annals of Thoracic Surgery.
During a CT scan, the radiologist will mark a nodule by
Winship Magazine | spring 2016 21
inserting a fiducial, which is then visible during the operation via fluoroscopy. This allows the surgeon to precisely cut out the appropriate wedge of lung tissue containing the nodule.
“Some nodules are small enough that it’s difficult to feel them at all,” Force says. “Rather than take as many as 45 minutes to hunt around for a nodule during surgery, this is an attractive and accurate alternative.”
Sometimes, cancer can be diagnosed and removed in one day. Some nodules are located deeper, so that it’s harder to access them by needle biopsy first.
A sample from a nodule can be removed during minimally invasive surgery, sent to the pathology lab, and within 30 minutes, the surgeon can have an answer to the question: is it cancer?

“We take out a small piece of lung tissue with a normal border, and put it in a bag so we don’t spill any tumor cells,” Pickens says. “If necessary, the patient can then have a definitive operation at that time through the small incisions.”
If the removed tumor is large enough, doctors will opt for a course of chemotherapy, even if no lymph node spread is detected. The critical measurement is how big the tumor actually is upon removal, rather than via imaging. The dividing line is four centimeters, set by nationwide studies that have examined the benefits of chemotherapy for earlier tumors.
Indiana trial attorney James Stankiewicz recalls having oncologist Fadlo Khuri explain that the tumor that Force had just removed from his lung was just above that threshold.
“We’re going to play it on the safe side,” he was told.
Like Huff, Stankiewicz also came to his diagnosis by a circuitous route—doctors in Chicago had diagnosed him with tuberculosis, before he asked for a needle biopsy to rule out cancer. When the biopsy came back positive, he came to Winship at the suggestion of a relative.
“Dr. Force was a reassuring voice during a dark, scary time,” Stankiewicz says.
Force was able to perform a lobectomy through an incision under one of Stankiewicz’s nipples. After surgery, he did experience some pain when moving his arms or twisting, he reports. Still, he was pleased with the results. He recently finished his cisplatin regimen and is scheduled for regular CT scans to continue monitoring his lungs.
“Ten years ago, we would have treated him much differently,” Force says. “We would not have given him chemotherapy.”
Outcomes research
Ongoing research continues in the lung cancer surgery field on whether open chest surgeries or minimally invasive approaches are best, as well as on the effectiveness of removing an entire lung lobe, versus partial lobe removal.
Emory surgeons Rachel Medbery, Felix Fernandez, and colleagues analyzed nationwide records and found that discovery of cancer in the lymph nodes was more common in open chest surgery (12.8 percent vs 10.3 percent), compared with video-assisted surgery. This suggests that open surgery may give the surgeon an advantage in performing a thorough lymph node inspection, although the difference was less for patients treated in an academic or research facility.

Fernandez is leading a push, supported by a grant in 2014 from the Agency for Healthcare Research and Quality, to compile more thorough information on lung cancer surgery outcomes. He and another leading thoracic surgeon in Florida are overseeing the integration of data compiled by the Society of Thoracic Surgeons (STS), which includes patient-level clinical details, with disease and mortality information from the Centers for Medicare and Medicaid Services.
“Because of the advances in lung screening, there’s a consensus that we’ll be seeing more patients with early-stage cancer who may be surgical candidates,” Fernandez says. “Minimally invasive approaches have a lot to offer, and it’s also an opportunity to define what will lead to better long-term outcomes.” W
22 Winship Magazine | winshipcancer.emory.edu feature | new strategies
A tiny camera is inserted through a laparoscope during minimally invasive surgery, allowing surgeons to see lung tissue on a video screen. Sancheti is able to remove a small sample of lung tissue for biopsy.
ERIC BERRY No. 29 Back in the Game
By Catherine Williams
Eric Berry has racked up accolades from the very beginning of his football career at Creekside High School in Fairburn, Georgia, throughout his years at the University of Tennessee, and now as a star safety for the Kansas City Chiefs.

But an award that recognizes Berry’s grit and determination both on and off the football field came in February when he was named the NFL’s Comeback Player of the Year.
Within the span of a year, Berry was diagnosed and treated for cancer, and then rejoined his team at summer training camp. By the second game of the season, Berry was once again a starter, and he finished the season selected to his third Pro Bowl.
to exercise throughout his therapy and maintain a high level of physical activity,” said Flowers.
The Winship nurses who cared for Berry were impressed with his attitude. “Eric came to each treatment with a will and determination to conquer cancer. Towards the end of treatment, we started a countdown, and he would dance with joy knowing that he was getting close to completion,” said nurse Stephanie Jones.
“Eric’s story and his strength are truly a testament to his individual willpower, his great spirit, his faith, and his supportive family. He is an inspiration to an incredible number of lymphoma and young adult cancer survivors. More than that, he is a great human being who has been very generous with his time to help our patients.”
C hristopher Flowers
When the diagnosis of Hodgkin lymphoma was made in December of 2014, Berry was back home with his family in Georgia. He came to Winship Cancer Institute for treatment and, under the care of lymphoma expert Christopher Flowers, underwent an aggressive chemotherapy regimen lasting into the spring. Berry’s determination and ability to stay in shape throughout treatment were extraordinary.
“This chemotherapy regimen is one where typically patients will be fatigued and have difficulty performing many of their usual activities in a regular daily desk job. It is very impressive that Eric was able to continue
Speaking on his first day back with the Chiefs on July 29th, Berry said, “The two things I could control were my attitude and my effort. I just tried to go out, wake up every day and try to build off whatever I did the day before. In that situation, anybody going through something like that, the only thing you can do is take it day by day.”
Berry says having the love and support of his family throughout treatment was a crucial part of his ability to stay positive. He’s thanked his mom and dad repeatedly for being by his side throughout. “When I lost my hair, Pops was right there. He shaved his head with me just so I wouldn’t be by myself.”
While his football career continues to surge forward, Eric Berry hasn’t forgotten what it’s like to be a patient. On a recent return trip to Winship for a checkup (and a happy reunion with his health care team), Berry also took time to visit two young patients currently battling cancer. W


Winship Magazine | spring 2016 23 feature | comeback player
KANSAS CITY CHIEFS
KAY HINTON
KAY HINTON
Game changers in philanthropy
By Marlene Goldman
Just nine months after her devastating diagnosis, Katie Ferraris Taylor lost her life to a rare form of leukemia. But in that short time, she cemented strong bonds with the oncology nurses who lovingly cared for her, laughed and cried with her, and celebrated her birthday and her wedding.
In 2007, she died at age 29 on the same note that she lived her life, says her father Garry Ferraris. “Katie was always upbeat and especially grateful to her nurses. She kept her sense of humor and never lost her sense of grace. When we would be weak she would be strong. She maintained a positivity that was unbelievable under the circumstances.”
The recent law school graduate experienced firsthand the vital role that oncology nurses play in improving the quality of life of patients and their families, and she


wanted to leave an enduring thank you. Her legacy is the Katie Ferraris Taylor Oncology Nursing Fund Award. Through the generosity of more than 200 donors, the endowment, now valued at some $142,000, has helped 30 nurses pay for continuing education.
Recent recipients Deatra Perkins, a 20year veteran of oncology nursing, and Catherine Caprara, a newly minted oncology nurse practitioner, share a passion for their work with cancer patients.
“There are still so many things to learn,” says nurse manager Perkins. “Oncology is a specialized area that is changing rapidly and requires nurses to have a lot of compassion and
strength for patients and families who are dealing with the most challenging time in their lives. With the scholarship I have the financial ability to increase my knowledge base, help the next patient, and share what I learn with my colleagues.”
Ferraris Taylor was a patient long before Caprara started her career on the oncology unit in 2012, but Caprara, who now works in bone marrow transplant, marvels at how “the Ferraris family keeps coming back to say thank you. Their gift has an effect not just on the scholarship recipients, but also on the countless patients we will serve. That’s the beauty of philanthropy: generosity perpetuates itself.”
24 Winship Magazine | winshipcancer.emory.edu giving back | making an impact
LEFT TO RIGHT: Deatra Perkins, one of four 2015 winners of the Katie Ferraris Taylor Oncology Nursing Fund Award; Garry Ferraris; Ginny Ferraris; Keith Taylor; award winners Heather Billings and Catherine Caprara. Not shown Carmen Hancock.
Paying It Forward
At Winship, acts of generosity are inspiring research, promoting prevention, and offering hope for healthier futures.
Consider Neil Gaines, who has raised $76,000 for Winship melanoma research.
“Skin cancer wasn’t anywhere on my radar when doctors removed an ugly mole on my back seven years ago,” says Gaines, who was then 23 and a recent University of Georgia graduate with a new job and a new love. “I didn’t know what melanoma was or its severity.”
The most common cancer in 25- to 29-year-olds, melanoma is more likely to spread to other parts of the body than other skin cancers. Gaines has endured five surgeries and multiple treatments. In his fifth year of remission from stage IV mela-
Sharing Expertise
The family of the late Surinder Puri is supporting cancer research by endowing an annual lecture to advance knowledge about B-cell malignancies, which include a variety of blood cancers. The lectureship attracts leading researchers and clinicians to share their work with professionals and patients and foster opportunities for multi-institutional collaboration.
Puri was a Renaissance man in the truest sense of the word, says Mridula Puri,
noma, he’s beating the average five-year survival rate of 15 to 20%.
Now married and a father, Gaines says that melanoma, if caught early, does not

screen and a hat, and visit their physician or dermatologist annually. His daughters, he insists, “will never use a tanning bed.”
Committed to supporting melanoma research, Gaines and his wife Margaret will host the eighth annual MelaNoMo this fall. The barbecue fundraiser on the Chattahoochee River has grown from a few family members and friends in 2008 to more than 160 people last year.
have to be deadly. He encourages others to look for signs of melanoma—a new spot on the skin; one changing size, shape, or color; or one that looks different—to wear sun-
“From the beginning I’ve wanted to use my story for the better good,” he says. “If I can help others understand the severity of this disease and the importance of education in melanoma, I can save lives.”
his wife of almost 45 years. An engineer, he was as interested in poetry, art, music, and cooking as he was in building bridges. He was instrumental in shaping Atlanta’s cityscape and infrastructure in the design and construction of MARTA.
But in 2004, a drooping eyelid led to a diagnosis of a stage IV mantle cell lymphoma, a rare blood disease. Through advances in research and clinical care at Winship, he thrived for more than a decade, working full-time and living a full life with friends and family he adored, until his relapse in 2014, and end of life in January 2015.
The inaugural Surinder K. Puri Memorial Lectureship on February 5, Puri’s birthday, featured Ash A. Alizadeh, from Stanford School of Medicine, discussing “Personalized Cancer Detection and Monitoring Using Deep Sequencing of Circulating Tumor DNA.”

The Puris have a long history with Emory, says Mridula Puri, a former faculty member. “We have great appreciation for all the faculty and staff, and our mission is to disseminate cutting-edge research and treatment information to benefit as many patients as possible.”
For more information about how you can support the work of the Winship Cancer Institute, contact 404-778-5175. W
Winship Magazine | spring 2016 25
Supporting Winship
MORE THAN 350 SUPPORTERS packed the halls of the Cherokee Town Club for the fifth annual Friends of Winship Fashion A Cure fashion show and luncheon. This year’s event was hosted by Monica Pearson, former WSBTV anchor and two-time cancer survivor, and




co-chaired by Kathy Bowman and Ree Edwards. Cancer survivors, caregivers, and friends modeled clothes from 19 local boutiques. The fundraising event brought in more than $170,000 in donations that will benefit Winship’s cancer research programs.
Pictured clockwise from top left (page 26 - 27):
1. Kathy Bowman and Ree Edwards, Fashion A Cure co-chairs.

2. Monica Pearson, Fashion A Cure emcee.

3. Rand Glenn Hagen, Lou Glenn and Louisa Glenn D’Antignac, Gala chairs.

4. Brenda Nease, Gala honorary chair, and David Lawson.
5. Wally Curran.
6. Mylin Torres and Malik Smith.
7. Suresh and Selvi Ramalingam.
8. Sagar Lonial and Ada Lee Correll.
26 Winship Magazine | winshipcancer.emory.edu giving back | the art of fundraising
12 8
Night & Day
THE FOURTH WINSHIP GALA on April 30 was the most successful fundraising event in Emory University’s history, raising more than $1.3 million to benefit cancer research at Winship. The Gala was chaired by Lou Glenn and







Winship Magazine | spring 2016 27
her daughters Rand Glenn Ha-
gen and Louisa Glenn D’Antignac. Brenda Nease served as honorary chair. More than 400 people attended the black-tie event at the Piedmont Driving Club. The Gala celebrated the compassion of patients, families, physicians, and researchers.
3 4 5 6 7 JIM FITTS
Navigating the language of cancer
 By Rebecca Pentz illustration by Rafael Lopez
By Rebecca Pentz illustration by Rafael Lopez
“I am persuaded that even with Google most patients enter a doctor’s office or hospital as if it were a Mayan temple, representing an ancient and mysterious culture with no language in common with the visitor.”—Tom
Brokaw,
If a regular visit to a doctor’s office is like entering a Mayan temple, understanding the terminology used in cancer treatment is like landing on Mars. We take this challenge of communication very seriously at Winship Cancer Institute.
For instance, chemotherapy terms can be difficult to understand, so the ethics team at Winship has interviewed 50 patients about those terms. In cancer, “stage” is not something for your kids and grandkids to perform on and “port” is not a nice after-dinner drink. But then what do they mean? Winship just awarded the ethics team a generous grant to develop an iPad slide show to explain this vocabulary, so that the language of chemotherapy will not be so foreign.
The idea of using an iPad program to explain important terms was pioneered by two of our prostate cancer physicians, Viraj Master and Ashesh Jani. After determining that many prostate cancer patients
have an extremely limited understanding of terms like impotence and incontinence, the doctors developed an iPad program using animation and simple language to explain them. Doctors use these and other terms to talk about the possible side effects of certain prostate cancer treatments, so understanding them is critical for patients facing decisions about their treatment options.
For cancers that have a hereditary component, Winship has a team of genetic counselors who can help you understand the impact of those genes on your treatment and the risk factors they might pose for your family. Although most cancers do not have a hereditary component, all tumors have genetic changes that tell the cancer cell to grow and spread. Doctors use terms like “driver mutations” and “mechanisms of resistance” when talking about these genetic changes. One of our
28 Winship Magazine | winshipcancer.emory.edu point of view
A Lucky Life Interrupted: A Memoir of Hope.
physicians, Suresh Ramalingam, explains that cancer is like a bus, and that there are certain genes that may be “out of whack” or mutated and thus in the driver’s seat. For some of these driver mutations, we have therapies that can stop the driver and his bus. Another Winship doctor, Walid Shaib, explains resistance like this: cancer travels on pathways, like I-75. We might be able to block I-75, but after a while the cancer often finds another pathway and heads up I-85 instead.
The ethics team has been listening to physician/patient conversations to find the best communication techniques to describe them. We will continue to sit in on these discussions and ask the patients afterwards if they understood the key terms. That way we can help other physicians better explain the genetics of cancer.
Clinical research studies also can be difficult to understand because they include both procedures that you would normally have for your treatment and
research-only procedures whose results have no impact on your care. You might have one blood draw early in the day that directs your treatment, but the very next blood draw goes straight to the research lab. To help you tell the difference, the ethics team gives each patient a chart with an X by every procedure in either the “helps me” or the “does not help me” box. We hope this helps patients understand the research that they so heroically participate in.
So yes, cancer is a Mayan temple, but at Winship you are not left to wander its dark hallways alone. We want to provide expert translators and knowledgeable guides for the journey.

Rebecca Pentz works with an innovative ethics research team to develop and design studies on ethical issues affecting cancer scientists, physicians, and patients, such as informed consent, biobanking, and genetic testing.

Winship Magazine | spring 2016 29


1365-C Clifton Road N.E. Atlanta, GA 30322 winshipcancer.emory.edu 1-888-Winship “ALMOST 25 YEARS AGO, I lost my wife, Allison, to breast cancer. She was only 37. We learned that when breast cancer hits young women, it is often very aggressive. Supporting cancer research helps fight this terrible disease, which is why I’ve named Winship a beneficiary of my life insurance policy. Making this gift honors Allison’s memory and helps Winship lead the way in finding a cure.” This is my legacy. Have you planned your legacy? giftplanning.emory.edu 404.727.8875 George Weaver Attorney
Georgia Printed by an FSC-certified printer using sustainable methods that reduce waste and volatile organic compound (VOC) emissions. Paper is a 50% PCW recycled sheet, sourced from a certified managed forest
Atlanta,






































































 By Rebecca Pentz illustration by Rafael Lopez
By Rebecca Pentz illustration by Rafael Lopez




